

The lower mantle consists predominantly of (Mg,Fe)SiO3 perovskite coexisting with about 20 vol% (Mg,Fe)O. Aluminum is a minor element in the lower mantle and is mainly accommodated in the perovskite phase. Previous studies show that the partitioning of Fe and Mg between perovskite and (Mg,Fe)O is strongly coupled to Al2O3 concentration, and the proportion of Fe3+ in perovskite may increase greatly if perovskite contains a small amount of Al2O3 (cf. 3.2d). If the conduction mechanism for perovskite is electron hopping from Fe2+ to Fe3+, the electrical conductivity of perovskite should depend on the concentration of Fe3+ and is thus sensitive to substitution of Al2O3 into perovskite. We test this hypothesis using in-situ electrical conductivity measurements of perovskite at lower mantle conditions.
We have measured the effect of alumina on the electrical conductivity
of (Mg,Fe)SiO3 perovskite transformed from two pyroxene samples
having similar iron contents: San Carlos orthopyroxene containing 3.3 wt%
Al2O3, and a synthetic orthopyroxene with a similar
iron content, (Mg0.915Fe0.085)SiO3. Samples
were first transformed to perovskite at 25 GPa and 1600°C in a multianvil
apparatus, and then prepared as disks for in-situ complex impedance spectroscopy
in a second experiment at 25 GPa and 1400-1600°C. Both the syntheses
and conductivity measurements were performed in the presence of a Mo-MoO2
buffer which maintains an oxygen fugacity close to the iron-wüstite
buffer at these conditions. Our results show that the activation energy
of 0.70 eV for conduction in perovskite containing Al2O3
is close to that of 0.62 eV for conduction in Al-free perovskite (Fig.
3.1-1). The electrical
 |
|
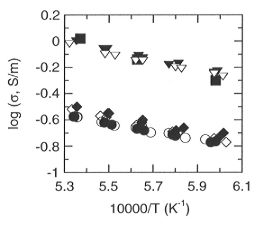
Fig. 3.1-1: Electrical conductivity of perovskite as a function of
reciprocal temperature at 25 GPa, 1400-1600°C. Squares and inverted
triangles are for Al-bearing perovskite H826 and H858, respectively, while
diamonds and circles are for Al-free perovskite H852 and H862, respectively.
Closed symbols refer to the first heating and cooling cycle and open symbols
refer to the second heating and cooling cycle.
|
conductivity of the Al-bearing perovskite is about 3.5 times that of
the Al-free perovskite. The Mössbauer results show that Fe3+/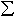 Fe in the Al-bearing perovskite (~ 42±
5%) is also about 3.5 times greater than that in the Al-free perovskite
(~ 12± 3%).
The similarity between the effects of Al2O3 on the
electrical conductivity and the amount of Fe3+ in perovskite
(the factor of 3.5) indicates that electrical conductivity of perovskite
is sensitive to the amount of Fe3+. This observation supports
the hypothesis that the conduction mechanism in perovskite between 1400°-1600°C
is most likely by polarons, for example, by electron hopping Fe2+->Fe3+ + e-
.
Fe in the Al-bearing perovskite (~ 42±
5%) is also about 3.5 times greater than that in the Al-free perovskite
(~ 12± 3%).
The similarity between the effects of Al2O3 on the
electrical conductivity and the amount of Fe3+ in perovskite
(the factor of 3.5) indicates that electrical conductivity of perovskite
is sensitive to the amount of Fe3+. This observation supports
the hypothesis that the conduction mechanism in perovskite between 1400°-1600°C
is most likely by polarons, for example, by electron hopping Fe2+->Fe3+ + e-
.
A conductivity-depth profile for the lower mantle can be constructed
from 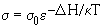 using the Al-bearing perovskite
parameters (Fig. 3.1-2). The results show that electrical conductivity
reaches a value of about 1 S/m at the top of the lower mantle, in agreement
with geomagnetic determinations.
using the Al-bearing perovskite
parameters (Fig. 3.1-2). The results show that electrical conductivity
reaches a value of about 1 S/m at the top of the lower mantle, in agreement
with geomagnetic determinations.
According to the expected change in conductivity for a temperature variation
of ± 100°C, our results do not favor
temperature increasing along the (Mg0.88Fe0.12)SiO3
perovskite adiabat at 660 km depth if the effects of Al2O3
on the conductivity of silicate perovskite are considered. Our results
are therefore consistent with whole mantle convection, rather than two-layer
convection involving a thermal boundary layer at 660 km depth.
|
|
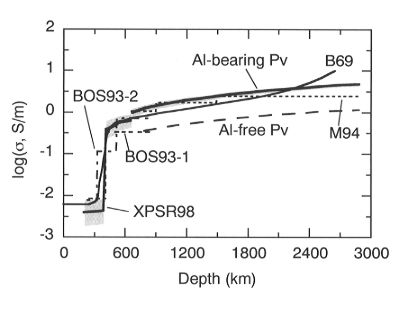
Fig. 3.1-2: Conductivity models for the lower mantle calculated
from the data for Al-bearing perovskite (thick line) and Al-free perovskite
(long dashed line) together with the upper mantle laboratory-based curve
XPSR98 (thick line) (Xu et al., Science, 280, 1415, 1998). Shaded areas
illustrate the effect on the model of a ±
100°C temperature variation. Geophysical models are shown as B69 (thin
line) (Banks, Geophys. J. Roy. Astr. Soc., 17, 457, 1969), M94 (dotted
line) (McLeod, J. Geophys. Res. 99, 13577, 1994), BOS93-1 (dashed line)
and BOS93-2 (dot-dash line) (Bahr, Olsen, Shankland, Geophys. Res. Lett.
20, 2937, 1993).
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page