

Several scenarios have been proposed for the formation of the Earth. The late veneer hypothesis is perhaps at the moment the most widely accepted theory. This theory stipulates that a late veneer of chondritic material was added to the Earth after core segregation. To test whether the Earth's core formed by simple chemical equilibrium between metal and silicate or whether the accretion was a complex heterogeneous process with many stages, we need to determine the metal-silicate partition coefficients DM/S for Highly Siderophile Elements (HSE) over a wide range of physical conditions (temperature, pressure, composition and oxygen fugacity).
The partition coefficients for some HSE have recently been determined at one bar. Values as high as 1012 were found for iridium at oxygen fugacities appropriate for core formation (log fO2= IW±1). The DM/S for Re, Pt and Rh have been found to be several orders of magnitude lower. Ultramafic rocks, believed to be representative of the upper mantle, contain significantly higher concentrations of highly siderophile elements than those deduced from these experimental values. Moreover, the contents of HSE in these mantle rocks are quite uniform. The large differences between partition coefficients of HSE and their extremely high values mean that present mantle compositions cannot be the consequence of metal/silicate equilibrium during core formation. The hypothesis of the addition of a late veneer of chondritic material to the Earth after completion of core formation appears more plausible. In order to investigate the problem further, partition coefficients for osmium and rhenium are being investigated.
An investigation of the partitioning of Os between metal and silicate melt is being made using the mechanically assisted equilibration technique at 1 atm. A crucible made of the metal is filled with silicate melt and brought to high temperature in a gas mixing furnace under reducing conditions controlled by a CO:CO2 atmosphere. A spindle made of the same metal is then introduced into the melt to stir it. The attainment of the equilibrium solubility of the metal in the silicate melt is demonstrated by a time series sampling. When equilibrium is reached, a change of the fO2, temperature and melt composition is possible without interrupting the experiment.
The crucible and the spindle used in this investigation have been made
by melting a Ni-Os powder mixture in alumina containers under very reducing
conditions. The microprobe analysis of the Ni-Os alloy shows an average
content of 5 wt% Os. The nickel solubilities in the melt can be used as
a comparative base for siderophile element behavior. The composition of
the silicate melt corresponds to the anorthite-diopside eutectic composition.
The initial conditions chosen for the experiment are T=1350°C and log
fO2 = -12. The glass samples are analysed by microprobe for
major elements (Si, Al, Ca, Mg), ICP-AES for Ni and ID-NTIMS for Os. Preliminary
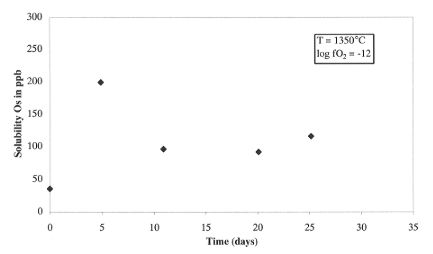
results indicate a low solubility of osmium in silicate melt (~
100 ppb) after one month duration (Fig. 3.2-3). These leads to a DM/S
value of about 107, which supports the model for the addition
of a late veneer to the Earth after core formation.
 |
|
|
It has also been suggested that the HSE partition coefficients might
decrease with pressure. Thus, it might still be possible to explain their
high concentrations in mantle rocks by a model of equilibrium core formation.
Consequently a further study has been initiated to investigate the effect
of high pressure on partitioning of HSE. The partitioning of Os and Re
between liquid metal and (Mg,Fe)O is determined as a function of pressure,
temperature and fO2 using a multianvil apparatus.
MgO capsules are filled with a Fe-Ni-Os-Re-O starting material and react
during the experiment to form (Mg,Fe)O (Fig. 3.2-4). The compositions of
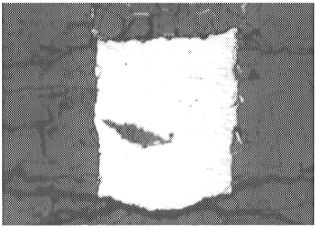 |
Optical micrograph of quenched liquid metal (light grey � 300 µm wide) in (Mg,Fe)O (dark grey). |
the quenched metal and (Mg,Fe)O (Mg, Fe and Ni) are determined by microprobe.
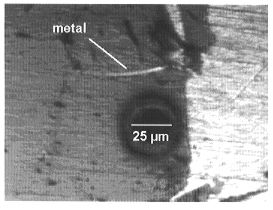
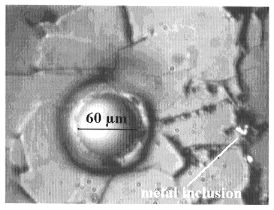
Re and Os in the (Mg,Fe)O are analyzed by LA-ICP-MS (Fig. 3.2-5).
 |
|
|
 |
|
The round spots in both photographs correspond to the laser ablation holes formed during analysis. |
This laser ablation technique was chosen because of its capacity for analyzing trace elements in situ. However, in the present study there are potential problems with this technique and considerable care is required. Because of the presence of thin films of quenched liquid metal along grain boundaries in (Mg,Fe)O, it is difficult to analyze this phase without contamination (Fig 3.2-5a). In addition, contamination of the sample surface during analysis is potentially problematic (Fig. 3.2-5b). These issues can be avoided by using a small spot size, but this increases the detection limit (e.g. with a 6 µm spot size the detection limit can be as high as 100 ppm). The concentration of Os in (Mg,Fe)O in the present experiments is below the detection limit and is estimated to be less than 10 pbb; this corresponds to a lower limit for the metal-oxide partition coefficient for osmium of about 107. The concentration of rhenium in (Mg,Fe)O is 1-6 ppm and gives a partition coefficient of about 104-105. These high liquid metal - (Mg,Fe)O partition coefficients for Os and Re provide additional support for the late veneer hypothesis.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page