

Results from high-pressure experiments combined with geophysical data
have indicated that majorite is likely a dominant phase in the transition
zone. Due to its variable oxidation state, iron is expected to play a major
role in determining the physical and chemical properties of majorite. As
a first step to characterize the effect of iron on majorite, we have examined
the system (Mg,Fe)SiO3. Samples were synthesised using a multianvil
press at varying oxygen fugacity conditions. All samples were examined
using the electron microprobe, Mössbauer spectroscopy, powder X-ray
diffraction and optical spectroscopy. Results show that Fe3+/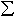 Fe
in (Mg,Fe)SiO3 majorite increases with both increasing oxygen
fugacity and total iron content. Rapid electron transfer occurs between
Fe2+ - Fe3+, likely across the shared edge between
the octahedral and dodecahedral sites. Fe3+ appears to occupy
the octahedral site primarily, and the amount of Fe2+ in the
octahedral site decreases with increasing Fe3+ content, coupled
with an increase in unit cell volume. We are using this data to construct
plausible defect models that balance the charge deficit caused by the substitution
of Fe3+ for Si4+.
Fe
in (Mg,Fe)SiO3 majorite increases with both increasing oxygen
fugacity and total iron content. Rapid electron transfer occurs between
Fe2+ - Fe3+, likely across the shared edge between
the octahedral and dodecahedral sites. Fe3+ appears to occupy
the octahedral site primarily, and the amount of Fe2+ in the
octahedral site decreases with increasing Fe3+ content, coupled
with an increase in unit cell volume. We are using this data to construct
plausible defect models that balance the charge deficit caused by the substitution
of Fe3+ for Si4+.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page