

Natural diamonds may form by a variety of mechanisms prevailing in different geological regimes. Besides the "classic" mechanism of formation in Earth´s mantle, radiation-induced formation in uranium-bearing carbonaceous materials, chemical vapor deposition (CVD), and martensitic shock transformation are recognized or, at least, have recently been suggested as additional formation mechanisms. Shock-transformed diamonds have been found in special groups of meteorites (ureilites, iron meteorites) and some terrestrial impact structures such as the Ries, Popigai, and Sudbury craters. The recent discoveries of diamonds in the Ries and at the K/T-boundary have triggered a debate on whether impact diamonds are formed by condensation and/or solid-state transformation. In this context, we report here the first discovery of impact diamonds in a fennoscandian crater, Lake Lappajärvi, a deeply eroded impact structure in Finland.
The diamond grains have been extracted from suevites and kärneites, i.e., impact melt breccias reflecting the highest degree of shock metamorphism. In order to understand their formation mechanism and subsequent thermal history, these grains have been studied by X-ray, optical and electronoptical (SEM and TEM) techniques.
Optical microscopy shows that the diamond grains have variable colour
(white, pale yellow, grey, and rarely black) and are thin flakes with diameters
< 100 µm and thicknesses < 20 µm. Debye-Scherrer X-ray
diffraction techniques reveal only one broad and continuous (111)-reflection
line of diamond at 2.06 Å. The continuity of the reflection line
suggests the polycrystallinity of the grains, whereas the absence of further
X-ray peaks and distinct X-ray line broadening both attest to the poor
crystallinity of the diamond aggregates. Detailed X-ray line broadening
analysis yields the small size of coherently diffracting diamond crystallites
in these grains with an average size of 280 Å. According to SEM observations,
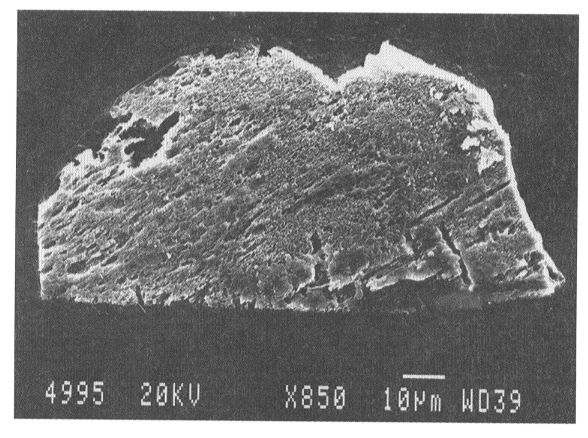
approximately 75% of the tabular diamond flakes show strong corrosion of
surfaces with a fine pitted relief (Fig. 3.4-2); the remaining fraction
exhibits smooth surfaces. Corroded grains additionally show distinct striations
on the surfaces, which probably are remnants of twin lamellae in the precursor
mineral graphite. At high magnification, individual small (< 100 nm)
diamond crystallites with a shape resembling elongated hexagons are visible
on the corroded surfaces. TEM observations corroborate the presence of
these diamond crystallites, arranged in the form of thin (< 100 - 200
nm) twin bands. In electron-transparent regions close to the original surface
of corroded diamond grains amorphous carbon is observed in addition. Diffuse
scattering in electron diffraction patterns and C K edge ELNES spectra
give evidence for the coating of diamond grains with amorphous carbon.
The ELNES spectra are characterized by the presence of the 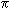 * peak at 285 eV, a signal which is absent in pure diamond.
* peak at 285 eV, a signal which is absent in pure diamond.
 |
|
Fig. 3.4-2: SEM image of a tabular diamond
grain from Lake Lappajärvi, Finland. The roughness of the surface
is due to corrosion in melt. Deep grooves are regions of pronounced corrosion.
|
In conclusion, the tabular morphology and inherited twin bands provide evidence for the direct solid-state transformation of these diamonds from graphite. The variation from uncorroded to corroded grains likely reflects the heterogeneous distribution in post-shock temperatures with peak temperatures in the melt particles and, hence, strong corrosion of diamond aggregates in melt inclusions of the impact breccias. The coating of corroded diamonds with amorphous carbon likely results from strong corrosion in the surrounding impact melt.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page