

In previous experiments with synthetic pure MgSiO3 enstatite, we showed that the incorporation of water is not necessarily coupled to chemical defects. However, water solubility increases in the presence of aluminium. In a new series of experiments, we measured water solubility in natural, pale-brown, gem-quality enstatite crystals from Tanzania. These crystals contained 1.5 wt% Al2O3 and 2 wt% FeO, among other minor impurities. The water content of the starting material was 214 ppm by weight, calculated from polarized IR-spectra. The crystals were annealed in the presence of a free hydrous fluid phase at 1100°C and 0.5 to 100 kbar. For the experiments above 10 kbars (piston-cylinder or multianvil press) we used a Ni-NiO-buffer to fix the oxygen fugacity. The runs in the low pressure range (0.5 - 2.0 kbar, TZM-bombs) were unbuffered with respect to oxygen fugacity. To determine water contents after the annealing experiments, polarized FTIR spectra were measured on oriented crystals. The water distribution inside the crystals was found to be homogeneous for run durations of several days.
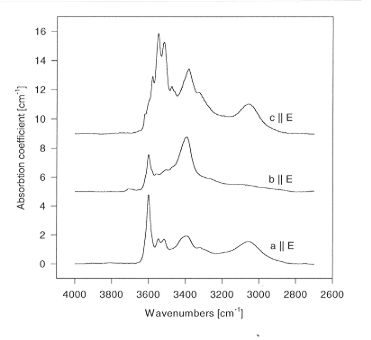
Polarized infrared spectra of annealed natural enstatite are shown in
Fig. 3.5-7. The spectra show additional bands if compared to pure MgSiO3.
However, there appears to be no major difference in the shape of the spectra
between buffered and unbuffered experiments. The absolute water solubility
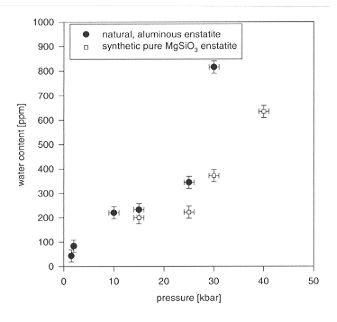
in the natural, aluminous enstatite appears to be somewhat higher than
for pure MgSiO3 (Fig. 3.5-8). According to our previous work,
this difference can be attributed to the presence of aluminium. The iron
content of the crystals and other trace impurities do not appear to significantly
affect water solubility.
 |
|
|
 |
|
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page