

Orthopyroxene with a composition near that of enstatite is one of the major minerals in the upper mantle of the Earth. Its physical and mechanical behaviour is expected to have significant influences on the properties of this region in the planet. In addition, the presence of trace amounts of water is known to have a major effect on the behaviour of olivine and probably also orthopyroxene. Consequently, in determining the physical properties of upper mantle minerals it is important to characterize carefully the effects of water. While it is frequently possible to perform experiments to measure properties under carefully controlled hydrous conditions, care must be taken to ensure that the mineral grains have attained equilibrium with the hydrous environment. This equilibration requires diffusion of hydrous species throughout the sample on the time scale of the experiment.
In order to characterize the time scales of equilibration of orthopyroxene grains with a hydrous environment we have performed experiments to measure the rates at which water-derived species diffuse within enstatite grains at high temperatures. The experiments were performed on natural iron-bearing enstatite samples from either San Carlos, Arizona, or Sri Lanka. The samples were prepared as rectangular prisms with sides of approximately 2-3 mm, and with faces perpendicular to the crystal axes of the orthopyroxene. All untreated samples contained significant amounts of hydroxyl (OH), as determined by infrared spectroscopy. We have performed three types of experiments: 1. Dehydration experiments on single crystals of enstatite under controlled oxygen fugacities (provided by mixed CO/CO2) at 1 atm and temperatures from 800 to 1100°C; 2. Hydration experiments in a piston cylinder apparatus on previously dehydrated samples of enstatite at 1 GPa fluid pressure, 800 to 1100°C, and oxygen activities buffered using Ni and NiO powders; and 3. One hydrogen-deuterium exchange experiment on an untreated sample at 1 GPa fluid pressure (provided by D2O), 900°C and with the oxygen activity buffered using Ni and NiO powders. The experimental durations were chosen such that the dehydration, hydration, or D-H exchange was only partial. In this way, diffusion of water-derived species was limited to the regions nearer the sample edges and the hydroxyl concentration in the center of the sample remained essentially unchanged.
After treatment the samples were cut into 3 slices. The central slice,
which was approximately 1 mm thick, was carefully polished on both sides
with 0.3 µm alumina powder for analysis using infrared spectroscopy.
Series of infrared spectra, such as those in Fig. 3.5-9, were taken
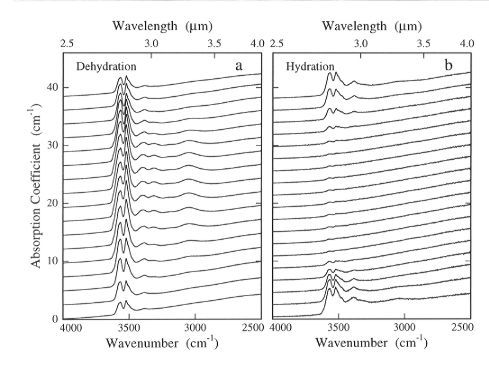 |
|
Fig. 3.5-9: Two series of infrared spectra taken as a function
of position parallel to the [001] axis of sample OPX 11-2 after a) an experiment
in which the sample was partially dehydrated at 1 atm for 5 hours at 1000°C,
and b) after the sample was partially rehydrated at 1 GPa for 10 minutes
at 1100°C following complete dehydration. Spectra that were taken near
the sample edges are shown at the lowermost and uppermost parts of the
figures and have significantly different hydroxyl concentrations than those
in the more uniform central region.
|
parallel to the two orthogonal crystal axes in the slice using polarised radiation with the electric vector parallel to the [001] direction. The beam diameter was 100 µm and the spacing between measurements was 100 µm near the edges of the sample and up to 300 µm nearer the middle. Integration of the OH bands in the infrared spectra yielded hydroxyl concentration profiles as a function of position in the sample. These profiles could then be fit using a solution to Fick´s laws for diffusion in a finite slab to yield diffusion coefficients for the mobile water-derived species in enstatite under the conditions of the experiments.
The rate of diffusion of water-derived species in enstatite was approximately the same for dehydration experiments, hydration experiments and the hydrogen-deuterium exchange experiment. The diffusion was also anisotropic, with diffusion parallel to [100] and [001] being fairly similar and about an order of magnitude faster than parallel to [010] in enstatite. A comparison of the absolute diffusion rates measured here for water-derived species and previously published data on diffusion of magnesium, oxygen and silicon in enstatite indicates that the water-derived species move many orders of magnitude faster than the other species in enstatite. In addition, the activation energy for diffusion is about 150 kJ/mol. The magnitudes of both the diffusion coefficients and the activation energies are similar to those from previous studies on diffusion of water-derived species in olivine and diopside. In these other minerals the diffusing species was determined to be protons, with transport of an electronic-defect species providing charge neutrality. The similar results for hydration/dehydration and hydrogen-deuterium exchange indicate that the diffusion of the protons is the rate-limiting step in all the experiments.
The results of these experiments show that water may be lost relatively rapidly from iron-bearing enstatite grains during transport from a mantle source region to the surface of the Earth. In addition, only relatively short times are required to sweep hydrogen into or out of enstatite during an experiment. On the other hand, full equilibration of hydroxyl in a sample requires the attainment of internal equilibrium of all defects, including hydroxyl, so that significant changes in hydroxyl concentration may take place on longer time scales than this initial rapid transport. In addition, as electronic defects are integrally involved in the transport process, samples with low iron concentrations, and consequently lower concentrations of electronic defect species, may yield significantly lower rates of diffusion for water-derived species.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page