

Moderately siderophile elements such as nickel and cobalt are believed to have been strongly fractionated from the mantle into the FeNi core of the Earth during its formation such that their abundances in the residual mantle reflect details of the core formation process. It is generally accepted that the depletion of siderophile elements results from their partitioning. However, it has long been known that their abundances in the Earth's upper mantle are far too high to have been produced by a simple equilibration of core-forming metal with mantle-forming silicate (although some volatile elements would have been lost). The direct determination of partition coefficients for such elements at high pressures and temperatures under well-constrained conditions remains experimentally challenging and thus, such efforts might be well complemented by high-pressure extrapolations from existing low-pressure partitioning data together with volumetric properties. Towards this end, we undertook a study to determine the partial molar volumes of NiO and CoO in silicate liquids.
The molar volumes of 7 Ni- and Co-containing sodium disilicate liquids (NiO- and CoO-contents range from 0 to 9 mol% and 0 to 23 mol%, respectively) have been determined over a large temperature interval by combining high-temperature measurements using the Pt-based double-bob Archimedean method and low-temperature measurements using a method described by Webb et al. in 1992 based on an assumed equivalence of the relaxation of volume and enthalpy at the glass transition. The molar volumes were fit using a regression equation from which the partial molar volumes of NiO and CoO were obtained. The results are reported in Table 3.6-1.
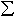 Xi [Vi,800 + (
Xi [Vi,800 + (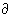 Vi/
Vi/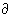 T)
(T-800)].
T)
(T-800)].| i | Vi,800 | 103 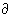 Vi/ Vi/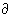 T T |
Vi,800 | 103 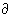 Vi/ Vi/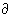 T T |
|
| NS2a | 24.968 (.022) | 2.758 (.0) | 25.004 (.043) | 2.519 (.1) | |
| CoO | 14.884 (.149) | 1.441 (.4) | --- | --- | |
| NiO | --- | --- | 11.506 (.687) | 2.684 (1.6) |
a The fit parameters for NS2 (sodium disilicate) obtained from both fits are also listed since we used this composition as an end-member
The change in the partition behavior of elements between coexisting metal and silicate phases as a function of pressure at constant temperature can be calculated based on the following equation:
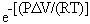 , (1)
, (1)in which DMmet-silT,1bar and DMmet-silT,P
are the metal-silicate partition coefficients of element M at temperature
T and 1 bar and at temperature T and pressure P, respectively. Possible
pressure dependence of the molar volume of MO (i.e., 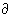 V/
V/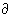 P)MO in the silicate liquid
has been ignored for simplicity.
P)MO in the silicate liquid
has been ignored for simplicity.
Experiments at one atmosphere have shown that the solubilities of metallic elements when dissolved as metal oxides in the silicate phase strongly depend on the oxygen fugacity that prevailed during equilibration. Therefore, the metal-silicate partition coefficients (i.e. the ratio of the mole fraction of the metallic element in the metal phase to the metal oxide in the silicate phase) also depend on oxygen fugacity. Taking this into consideration, DMmet-sil can be expressed relative to DFemet-sil in Fe-containing systems by using the M-Fe metal-silicate exchange partition coefficient KDM-Femet-sil where
The pressure effect on KDM-Femet-sil is analogous to equation 1, so that:
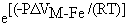 . (3)
. (3)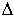 VM-Fe is the change in the molar
volume of MO for the M-Fe exchange reaction (i.e., VMO-VFeO).
If the partial molar volumes of NiO, CoO and FeO liquids are not too different
from the molar volumes of their pure liquids, then the molar volumes of
pure MO liquids (i.e., NiO and CoO) and pure FeO liquids can be determined
using their partial molar values listed in Table 3.6-1 and that given by
Lange and Carmichael in 1987 for FeO. As a caution, however, the molar
volumes of these pure liquids may be different to their partial values
since they result in large extrapolations.
VM-Fe is the change in the molar
volume of MO for the M-Fe exchange reaction (i.e., VMO-VFeO).
If the partial molar volumes of NiO, CoO and FeO liquids are not too different
from the molar volumes of their pure liquids, then the molar volumes of
pure MO liquids (i.e., NiO and CoO) and pure FeO liquids can be determined
using their partial molar values listed in Table 3.6-1 and that given by
Lange and Carmichael in 1987 for FeO. As a caution, however, the molar
volumes of these pure liquids may be different to their partial values
since they result in large extrapolations.
As shown by equation 3, the change of KDM-Femet-sil
as a function of pressure, i.e. increasing or decreasing partition coefficient,
depends only on 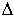 VM-Fe. The metal-silicate
exchange partition coefficient of Ni, KDNi-Femet-sil
will increase with increasing pressure at temperatures lower than 2320
K and decrease with increasing pressure at temperatures higher than 2320
K, whereas the metal-silicate exchange partition coefficient of Co, KDCo-Femet-sil,
will decrease with increasing pressure even at temperatures as high as
3000 K. We summarize these effects in Table 3.6-2.
VM-Fe. The metal-silicate
exchange partition coefficient of Ni, KDNi-Femet-sil
will increase with increasing pressure at temperatures lower than 2320
K and decrease with increasing pressure at temperatures higher than 2320
K, whereas the metal-silicate exchange partition coefficient of Co, KDCo-Femet-sil,
will decrease with increasing pressure even at temperatures as high as
3000 K. We summarize these effects in Table 3.6-2.
| T(K) | Vliq(MO) | 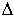 VNi-Fe VNi-Fe |
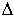 VCo-Fe VCo-Fe |
P dependencea of KDM-Femet-sil | |
| Ni | Co | ||||
| 800 | V(NiO) < V(FeO) < V(CoO) | -0.102 | 3.261 |  |
 |
| 1473 | V(NiO) < V(FeO) < V(CoO) | -0.057 | 2.509 |  |
 |
| 2350 | V(FeO) < V(NiO) < V(CoO) | 0.002 | 1.530 |  |
 |
| 3000 | V(FeO) < V(NiO) < V(CoO) | 0.046 | 0.804 |  |
 |
a : V(MO) < V(FeO),
KDM-Femet-silT,P > KDM-Femet-silT,1bar,
KDM-Femet-sil will increase with increasing pressure
: V(MO) < V(FeO),
KDM-Femet-silT,P > KDM-Femet-silT,1bar,
KDM-Femet-sil will increase with increasing pressure
 : V(MO) > V(FeO), KDM-Femet-silT,P
< KDM-Femet-silT,1bar, KDM-Femet-sil
will decrease with increasing pressure.
: V(MO) > V(FeO), KDM-Femet-silT,P
< KDM-Femet-silT,1bar, KDM-Femet-sil
will decrease with increasing pressure.
The pressure dependence of NiO and CoO solubilities in FeO-containing silicate melts has been recently reported in the literature. All of these experimentally determined Ni-Fe and Co-Fe metal-silicate exchange partition coefficients, calculated using equation 2, show a decrease with increasing pressure at constant temperature, and this is independent of silicate and metal compositions. This is in good agreement with the qualitatively predicted pressure dependencies of KDNi-Femet-sil and KDCo-Femet-sil for temperatures higher than 2320 K as calculated by equation 3, which is based on the partial molar volumes of NiO and CoO in silicate melts.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page