

The processes involved in planetary accretion and core formation are subject to considerable debate, which is generally argued in the literature as homogeneous versus heterogeneous accretion. The first possibility describes a unique process with a single kind of accreting material. The result would be a global equilibrium at some P, T, and fO2 between core and mantle, in contrast to the case of heterogeneous accretion where variable P, T and fO2 could coexist locally. Until now no unique solution of P, T, and fO2 has been found which adequately explains the abundance of the siderophile elements in the earth's interior.
Most metal-silicate distribution coefficients have been derived at 1 atm. In the last few years, however, experiments have been undertaken at elevated pressure. Results from the high-pressure studies have indicated a non-neglible pressure dependence of the distribution coefficients. However, such studies are experimentally quite difficult and interpretation of their results have often been complex. A complementary or alternative strategy is to derive the pressure dependence of partitioning from the volumes of the exchange components in the melt and metal phases. Equations expressing the pressure dependence of the distribution coefficients and two-element distribution coefficients that avoid difficulties with unknown oxygen fugacities have been given in the previous section of this Annual Report (3.6e) by Courtial et al.
As we are dealing with liquids, the partial molar volume of the metal oxide in the silicate liquid is required. The density of a silicate liquid is given by:
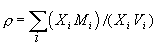
where Mi= molecular weight of i; Vi=partial molar volume of i. It is possible to calculate partial molar volumes of oxides via measured densities of melts for which the oxide of interest has been systematically varied in concentration. The most precise method is the double bob Archimedean method that makes use of replicate measurements of the buoyancy of two different platinum bobs such that the density can be calculated by:
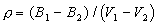
where Bi = buoyancy of bob i.
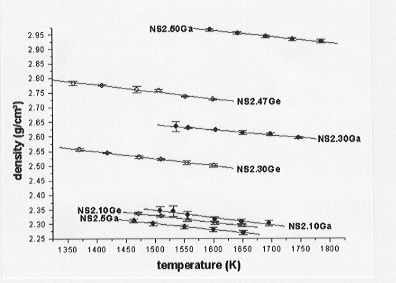
Towards this end we have been engaged in the determination of the partial molar volumes of Ga2O3 and GeO2 in sodium disilicate (Na2Si2O5) melts. Within the temperature range investigated, densities range from 2.27 to 2.95 g/cm3 for the Ga-bearing melts and from 2.30 to 2.80 g/cm3 for the Ge-bearing melts (Fig. 3.6-4). Furthermore, the Ga-bearing melts exhibit
 |
|
|
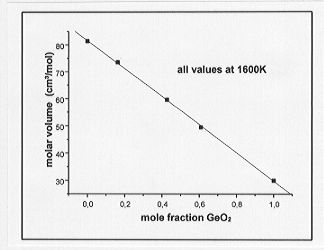
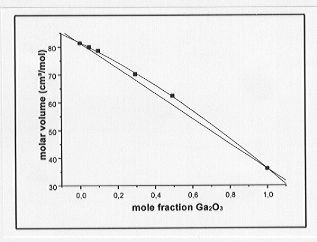
a greater density than the Ge-bearing melts at equivalent molar concentrations and temperatures. Molar volumes of the melts are plotted as a function of metal concentration in Fig. 3.6-5 and Fig. 3.6-6 for the Ge- and Ga-bearing melts, respectively. The molar volume of the Ge-bearing melts decreases linearly with increasing Ge content whereas a nonlinear relationship is observed for the Ga-bearing melts. A possible interpretation for the latter observation could be that a coordination change of Ga occurs with varying composition in the melt.
It is further planned to perform measurements with the anorthite-diopside
eutectic composition as the silicate endmember in order to see if similar
trends can be observed and if the partial molar volumes of Ga2O3
and GeO2 are independent of the silicate endmember, at least
at geologically relevant concentrations.
 |
|
|
 |
|
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page