

The partitioning of nickel between melt and crystals has long been investigated in the earth sciences due to the importance of Ni trace element chemistry in evaluating scenarios of basalt genesis. The effects of both temperature and melt composition on the partitioning of Ni therefore must be understood. The derivation of nickel activities from partitioning experiments involving near liquidus equilibration between crystals and melts yields data which unavoidably combine the influences of both temperature and composition. Metal-melt partition coefficient studies, in contrast, provide the possibility of independently obtaining data on the temperature and melt compositional dependencies of Ni activity. Ertel et al. (1997) studied the melt composition effects on the Ni partition coefficient in haplobasaltic melt (synthetic melt having a similar composition to a basalt). The technique used was the mechanically assisted equilibration technique described in section 3.2b of this Annual Report. They showed that varying the forsterite and quartz components has no influence on nickel solubilities in the silicate melt, whereas the Na-metasilicate component (Na2SiO3) generates a complex compositional dependence that can be explained by considering the structural role of Ni in the melts. To further understand the compositional dependence of Ni activity and the influence of melt structure, the effects of Li, Ca, and K-metasilicate components on Ni solubilities in 1 atm anorthite-diopside eutectic composition melt have been studied. The technique and conditions were the same as in the study of Ertel et al.
Nickel analyses in the glass were obtained by electron microprobe. The
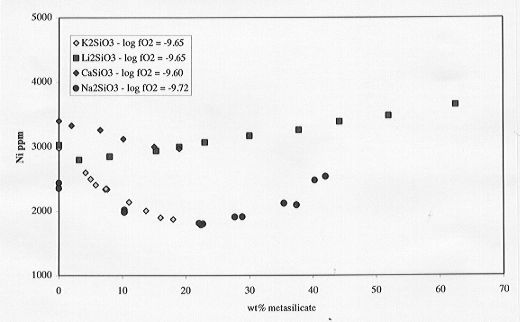
results of those analyses are shown on Fig. 3.6-7. A decrease in Ni solubility
is observed with addition of Ca and K metasilicate (up to 20 wt%) similar
to that observed for low concentrations of Na metasilicate. Addition of
Li metasilicate results in a similar composite effect as observed for Na
metasilicate but with the minimum in Ni solubility shifted to very low
contents (4 wt% Li metasilicate).
 |
|
Fig. 3.6-7: Compositional influences of Ca, Li, Na, K metasilicates
on Ni solubilities in silicate melt (anorthite-diopside 1 atm eutectic
melt composition).
|
Comparison of the trend observed for the alkali metasilicates leads to the interpretation that the relative stability of network stabilizing alkali aluminate complexes is K>Na>Li. This trend is consistent with a number of other sources of structural and property data such as the one proposed by Galoisy and Calas (1993) that "the higher the cation field strength, the less efficient the charge compensation".
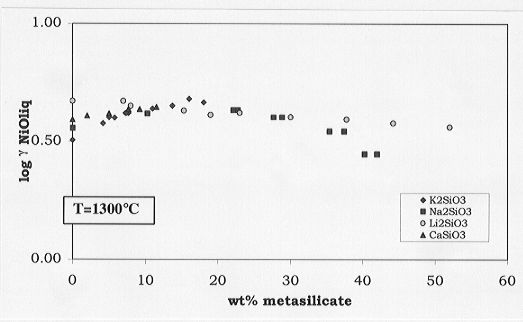
From these data, the activity variations of the nickel solubilities
with respect to the K, Ca, Li or Na metasilicate concentrations can be
deduced (Fig. 3.6-8). No significant variations in the activity coefficients
with addition of metasilicates are observed, leading to the conclusion
that melt composition plays a relatively minor role for determining the
melt-olivine partitioning. Other factors, amongst them temperature, are
expected to play a larger role. This work shows that siderophile elements
appear to be, on the one hand, very fine monitors of the melt structural
consequences of compositional variations. On the other hand, they may ultimately
be a highly precise source of activity data.
 |
|
Fig. 3.6-8: Effects of Ca, Li, Na, K metasilicates on the activity
coefficients of NiO in anorthite-diopside 1 atm eutectic melt.
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page