

Our understanding of the influence of pressure on the structure and
properties of silicate melts has been advanced greatly in the last few
years via a combination of large volume syntheses in multianvil apparatus
with solid state NMR spectroscopy. Additionally, recent diffusivity studies
have indicated that composition dependent parameters such as network polymerization
and Al/(Alt+Si) ratio are very important as to whether dynamic properties
such as melt viscosity will or will not be sensitive to changes in pressure.
Most of the studies leading to these conclusions, however, have been limited
to a narrow range of composition space and even narrower sampling of network
polymerization. In particular, most of the structure property studies of
melts and glasses at pressures above 2 GPa have focussed on very highly
polymerized alkali silicate glasses (with NBO/T=0.5) and even higher polymerized
alkali aluminosilicates (e.g. albite). We have synthesized about twenty
glasses in the CaO-MgO-Al2O3-SiO2 system
quenched isobarically at pressures up to 10 GPa from temperatures as high
as 2300° C using the multianvil apparatus.
Compositions range from simple depolymerized binary silicates (e.g. CaSiO3)
to more complex polymerized aluminosilicates (e.g. Ca2MgAl2Si4O14).
Examination of the run products by optical microscopy shows that they are
transparent and glassy with no signs of quench crystallization. Raman spectra
have now been obtained for several samples and show smooth, continuous
changes as a function of pressure (Fig. 3.6-11). Although Raman spectroscopy
is not as ideal as NMR spectroscopy in identifying and quantifying different
network forming cation coordination species, we suspect that with increasing
pressure both five- and six-coordinated silicon and/or aluminum may be
present in these glasses, particularly those which are only slightly depolymerized.
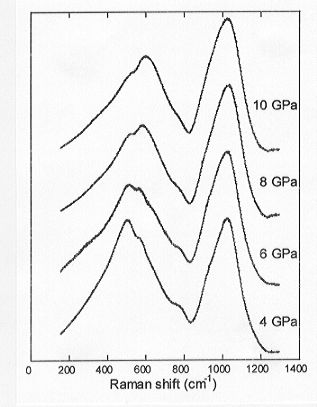
As shown in Fig. 3.6-11, the Raman spectra do indicate clear changes in
the anionic framework structure
 |
|
Fig. 3.6-11: Raman spectra of calcium aluminosilicate glasses
obtained by quenching isobarically from the melt phase at 2200°
C and the pressures indicated. Composition of the glasses are all 26% CaO
+ 9% Al2O3 + 65% SiO2.
|
of the melt, which can be attributed to a decrease in the average intertetrahedral T-O-T bond angle with increasing pressure (broad low frequency band centered near 500 cm-1 that shifts to higher frequency). The spectra show no indications that pressure influences the distribution of Q-species as the high frequency portion of the spectrum remains virtually unchanged. Further analysis of the Raman data as they are obtained should provide a more comprehensive interpretation of both the effect of pressure on the structure of silicate melts and how chemistry (e.g. Ca-Mg and Al-Si substitution) affects melt structure at high pressure.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page