

The density of silicate liquids is a fundamental parameter affecting the buoyancy of magmas. Most density measurements have been conducted at relatively high temperatures and low viscosities, necessitating large extrapolation over a vast temperature range in which critical structural changes may occur (e.g. close to the glass transition) that are critical in magma processes. To address this discrepancy, Webb et al. (European Journal of Mineralogy 4, 95-104, 1992) adopted a different approach based on the testable assumption of similar relaxation times of volume and enthalpy. By combining both calorimetric and dilatometric investigations on geologically relevant silicate melts they quantified melt densities within the super cooled liquid state. However, this method has yet not been accepted generally.
A totally different approach to quantify liquid densities within the
super cooled liquid state is provided by container based dilatometry, where
the thermal expansivity is measured directly. This new approach is entirely
free of assumptions regarding relaxation processes. Figure 3.8-7 shows
the experimental assembly, which is contained in a NETZSCH 402 TMA dilatometer
in vertical geometry. It consists of a hollow right cylinder in which the
sample and two metal end pieces reside. A quartz push rod rests on the
upper end piece and moves according to the expansivity of the sample as
the temperature is changed. To calculate the thermal expansivity, expansion
of both the hollow cylinder and end pieces also must be taken into account.
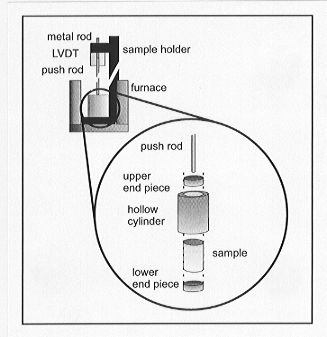 |
Fig. 3.8-7: Schematic illustration of the container assembly
used for the determination of liquid thermal expansivity. The container
is machined from Ni-metal. Its inner dimensions precisely match those of
the sample cylinder at room temperature. Movement of the upper end piece
records the net expansion of the sample and the container as the sample
is heated. |
We used a standard soda-lime glass (NIST 710 SRM) to test the new method.
The constrained sample was heated at a set rate of 5 K/min to 180K beyond
its glass transition temperature (Tg = 583°C) in order to
ensure that the sample completely filled the container space. Measurements
were taken during cooling at a rate of 5 K/min. Upon cooling the container
walls remain wetted allowing the direct determination of the sample volume
at any temperature within the super-cooled liquid state. To test the accuracy
of the new technique we also applied the method of Webb et al. (1992) to
derive the thermal expansivity of the sample within the supercooled liquid
state. As shown in Fig. 3.8-8 both data sets reveal excellent agreement.
Future work will include the direct determination of liquid expansivities
of geologically relevant melts at ambient as well as at elevated pressures.
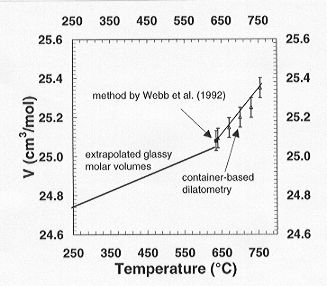 |
Fig. 3.8-8: Comparison of expansivity data derived using
the new method (open triangles) with that of Webb et al. (1992) (square),
including an extrapolation of their expansivity data to higher temperatures. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page