

Given that the presence of water in a rhyolitic melt can substantially alter such important characteristics as viscosity and diffusivity, a large body of work has been built up on the speciation of water in such systems. Previously, these studies have used simple analogues such as haplogranite, and have concentrated on hydrous glasses. Recently, attention has focused more on in-situ techniques, and we are now in a position to study systems closer to natural melt compositions.
Samples of hydrous rhyolitic glass have been investigated at both high temperature and pressure, using a hydrothermal diamond anvil cell. The design and usage of this cell allows for the accurate determination of water speciation using FTIR spectroscopy under purely hydrostatic conditions, and with no interference from the pressure medium.
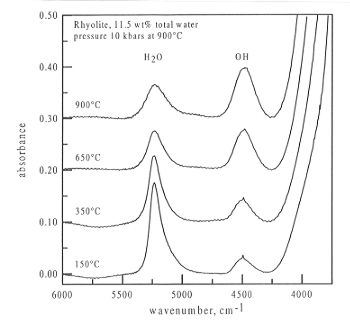
Figure 3.5-10 shows a series of spectra obtained from a sample of rhyolite
containing 11.5 wt% total water at a maximum pressure of 10 kbar. As can
be clearly seen, the peak at 4500 cm-1, due to OH groups increases
in intensity, whereas the peak at 5200 cm-1, due to molecular
water, decreases in intensity with temperature. As previous studies performed
at the Geoinstitut have shown that the extinction coefficients for both
these peaks do not change with temperature, this represents a real speciation
change with temperature.
 |
|
Fig. 3.5-10: Infrared spectra obtained from a sample of rhyolite
in the diamond anvil cell at 150, 350, 650, and 900°C. The pressure
medium had a density of 0.85 g cm-3 giving a pressure of 10
kbars at 900°C. The sample thickness was 97 µm.
|
The speciation reaction can be written in the form:
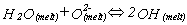
and it is therefore possible to write an equation to obtain the equilibrium constant, k for the reaction:
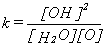
where [OH], [H2O] and [O] are the concentrations of the three
components in the reaction. This can be used to obtain further thermodynamic
information on the speciation reaction by plotting the ln k versus reciprocal
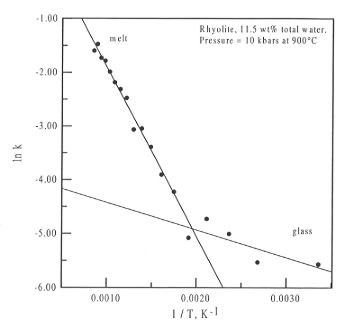
temperature in K, and this is shown in Fig. 3.5-11 for the sample in Fig.
3.5-10.
 |
|
Fig. 3.5-11: Plot of ln k versus 1 / T for the sample in Fig.
3.5-10. The separation of the data into two groups, one for the glass phase,
the other for the melt, can be clearly seen. The intersection of the two
fitted curves gives the glass transition temperature for the system.
|
As can be clearly seen, the data fall into two parts, one above the glass
transition temperature, Tg, and one below. The intersection
of the curves fitted to the data allows for the determination of Tg
for the experiment, in this case 525 K, and both 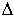 H and
H and 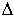 S for the reaction
can be obtained from the equations of the fitted curves. For the melt phase
(where the equation of the fit is lnk = - 3279.78 * 1/T + 1.379),
S for the reaction
can be obtained from the equations of the fitted curves. For the melt phase
(where the equation of the fit is lnk = - 3279.78 * 1/T + 1.379), 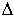 H is
equal to 27.27 kJ mol-1 and
H is
equal to 27.27 kJ mol-1 and 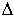 S is equal to 11.46 J mol-1.
This confirms that major changes in the speciation occur above Tg.
There is possibly also some change in speciation below the glass transition.
However, this latter fact cannot be confirmed, as the variation in the
data is probably on the same order as the experimental uncertainty.
S is equal to 11.46 J mol-1.
This confirms that major changes in the speciation occur above Tg.
There is possibly also some change in speciation below the glass transition.
However, this latter fact cannot be confirmed, as the variation in the
data is probably on the same order as the experimental uncertainty.
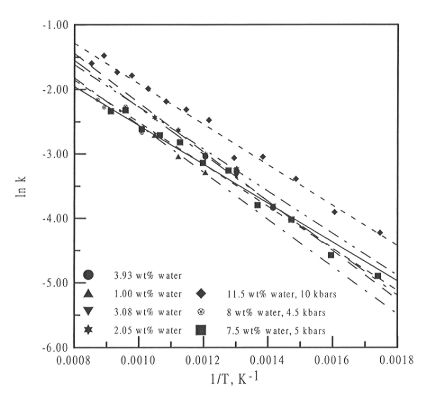
Figure 3.5-12 plots ln k versus reciprocal temperature for the melt
phases for three different diamond cell experiments, at 10, 5 and 4.5 kbars,
and also plots the data from the high temperature studies at 1 bar reported
last year. All of the fits are parallel or sub-parallel, and there is no
systematic variation in the values of  H and
H and  S that can be obtained. Therefore,
it seems that over the pressure range studied, pressure has a negligible
effect on water speciation in silicate melts.
S that can be obtained. Therefore,
it seems that over the pressure range studied, pressure has a negligible
effect on water speciation in silicate melts.
 |
|
Fig. 3.5-12: Plot of ln k versus 1 / T for the melt phase of
three different high pressure experiments and for the high temperature
data obtained previously.
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page